Kids 6 Months To 5 Years Are Now Eligible For The Updated Covid-19 Vaccine
The bivalent vaccines target the original strain as well as the BA.4/5 Omicron strains.
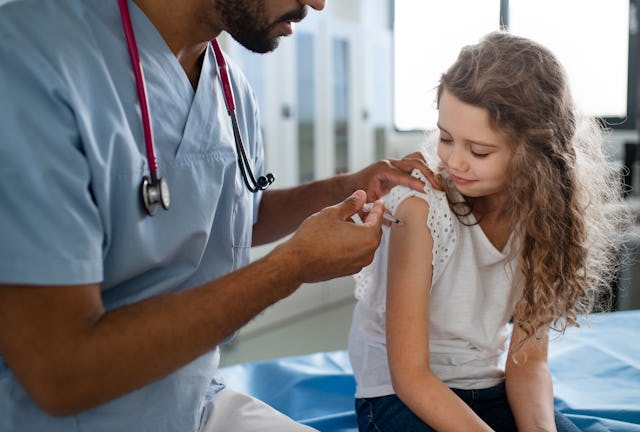
The US Food and Drug Administration (FDA) announced on Thursday the authorization of an updated COVID-19 vaccine from Moderna and Pfizer/BioNTech to be used in children from ages 6 months through 5 years.
The bivalent vaccines target the original strain as well as the BA.4/5 Omicron strains. These vaccines were previously authorized as a booster dose only for people age 5 and older.
In a statement from the FDA, they encouraged parents to get this updated vaccine to protect their children during these especially harsh winter months.
“More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to consider doing so — especially as we head into the holidays and winter months where more time will be spent indoors,” FDA Commissioner Dr. Robert Califf said.
While this is great new for worried parents, there are some stipulations when it comes to who can receive this updated vaccine and when they can receive it:
- Children age 6 months through 5 years old who received the original, two-dose Moderna vaccine are eligible to receive a single booster of the updated bivalent Moderna vaccine two months after completing the primary vaccine series.
- Children age 6 months through 4 years who haven’t started the vaccine process and plan to go with Pfizer will received the updated vaccine as their third dose in the Pfizer primary vaccine series.
- Children ages 6 months through 4 years who have completed the three-dose primary series of the original Pfizer vaccine are not eligible for an updated booster dose at this time.
While some parents may be hesitant about an updated vaccine for their kids, the FDA assures that keeping updated on vaccination status is the best way to combat COVID-19.
“Vaccines remain the best defense against the most devastating consequences of disease caused by the currently circulating omicron variant, such as hospitalization and death. Based on available data, the updated, bivalent vaccines are expected to provide increased protection against COVID-19,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research, in the FDA’s official statement.
“Parents and caregivers can be assured that the FDA has taken a great deal of care in our review, and we encourage parents of children of any age who are eligible for primary vaccination or a bivalent COVID-19 vaccine booster dose to consider seeking vaccination now as it can potentially help protect them from COVID-19 during a time when cases are increasing.”
Read the entire statement here for all the details on the updated vaccine.