Pfizer Will Seek FDA Approval For A Maternal RSV Vaccine This Year
Initial trail results for the vaccine showed very promising results.
Now that COVID-19 restrictions are lifted in most places and life is somewhat “back to normal,” we’re all back to being at risk for getting some sort of sickness, COVID or otherwise, especially with school back in session. Currently, hospitals across the country are overwhelmed with kids suffering from respiratory illnesses as we head into fall and winter.
One of the worst illnesses that is spreading like wildfire has been RSV (respiratory syncytial virus), a respiratory virus that can be especially harsh and even dangerous in babies and young children. Due to the perfect storm of loosened COVID restrictions and two years of people wearing masks, social distancing, and staying healthy, RSV has been showing signs of coming back full force — and more — since June.
It may seem like your kid is sick every other week from something, or maybe you’re worried about RSV and how it could affect your child if they become infected. There could be some hope on the horizon to help ease that stress.
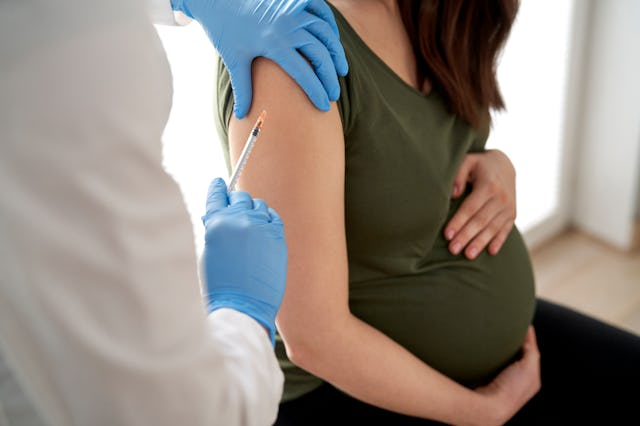
Pfizer says it has enough promising data on its RSV vaccine that it will end enrollment in the study currently going on and submit approval from the FDA (Food and Drug Administration) approval by the end of the year. Pfizer’s vaccine candidate would be administered to pregnant women who then make antibodies that cross the placenta and protect the baby after birth.
“The [study data monitoring committee] recommended, based on the data that we have, that we should go ahead and file, that this offers the potential for a safe and effective vaccine that could really dramatically help to prevent RSV during the winter season,” Dr. William Gruber, Pfizer’s senior vice president of vaccine clinical research and development, told CNN.
The trial showed to be about 80% effective at preventing severe RSV disease in infants in the first three months of life after their mother received the vaccine. It also cut a baby’s risk of needing to see a doctor for an RSV infection by half.
According to CNN, Pfizer’s vaccine contains the F-protein found in RSV, which the virus uses to attach to human cells. The protein is frozen into the shape it folds into before it fuses with a cell, so the immune system can build antibodies against it. Pfizer’s RSV vaccine is bivalent, containing F-proteins from both the A and B subgroups of the RSV virus, which are the two most commonly circulating strains.
Typically, RSV is prominently seen in the fall, winter, and spring months. However, there were already signs that RSV way back in the summer. The CDC released a memo on June 10th saying that RSV was already spiking in the South, and urged doctors to start testing kids for RSV if they presented with respiratory symptoms but had tested negative for COVID.
The CDC warned that older babies and toddlers could be especially hard hit with RSV since most kids are exposed to it at least a few times during childhood and build up some immunity to it. Not having been exposed to RSV for the past two years or so might mean that kids who get it for the first time have more serious cases.
The CDC was definitely onto something considering reports of thousands of kids in the ER and in hospitals due to respiratory viruses until some units are at capacity. In fact, RSV cases tripled for people of all ages in September alone — and cases have been more severe than usual, landing a high number of kids in hospitals struggling to breathe. The virus usually peaks in January or February.
If the FDA approves the maternal vaccine, Pfizer will have created the first inoculation against RSV and the first new product related to the infection in over twenty years.
With the FDA designating Pfizer’s RSV vaccine a breakthrough therapy, the speed of its review can fast track.
“That puts us in a very good position to essentially have something well in advance of next winter,” Gruber said, noting that both Pfizer and the FDA are conscious of the ongoing heavy RSV season in the US.