WHO Stops Study Of Hydroxychloroquine Trial Over Safety Concerns
The study was published in The Lancet late last week
The World Health Organization is pausing clinical trials that use hydroxychloroquine to treat COVID-19 patients after a medical journal published a paper last week stating patients taking hydroxychloroquine were dying at higher rates than those who were not.
The Lancet reported that, not only did researchers not observe “any benefit of hydroxychloroquine or chloroquine (when used alone or in combination with a macrolide) on in-hospital outcomes,” but that “ventricular arrhythmias were more common and mortality was higher” in the groups that took the drug compared with the control population.
The WHO, which has 3,500 patients from 17 countries enrolled in a “Solidarity Trial,” are looking at new treatments to help fight COVID-19 in patients who were diagnosed, and were using hydroxychloroquine as a treatment in some patients. Once The Lancet published their findings, the WHO halted the part of the trial using the drug, but are continuing with other treatment options.
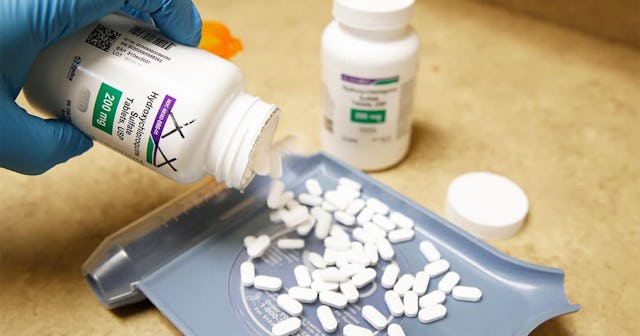
Hydroxychloroquine is an immunosuppressive drug and anti-parasite used to treat and prevent malaria. It can also treat lupus and arthritis.
Recently, Donald Trump told reporters he began taking hydroxychloroquine to protect himself from the coronavirus, not heeding warnings from his own government that the drug has not been shown to be effective in treating or preventing the coronavirus, and that it can have potentially fatal side effects.
Trump began mentioning the “miracle cure” weeks ago during his daily press conferences because of its antiviral properties that make it effective in preventing malaria. A number of clinical trials have tested the drug against COVID-19, and there were no findings to support those claims. Now, it seems, it could have the opposite effect.
Since then, the sales of hydroxychloroquine doubled from March 2019 to more than $50 million in March of this year, according to market research firm IQVIA, which tracks prescriptions given by retail pharmacies.
According to the paper, researchers analyzed data from more than 96,000 patients with confirmed Covid-19 from 671 hospitals. Just under 15,000 patients were treated with hydroxychloroquine or chloroquine, or one of those drugs combined with an antibiotic. About one in 11 patients in the control group died in the hospital, and approximately one in six patients treated with chloroquine or hydroxychloroquine died in the hospital. About 1 in 5 treated with chloroquine and an antibiotic died and almost 1 in 4 treated with hydroxychloroquine and an antibiotic died.
“The review will consider data collected so far in the Solidarity Trial and in particular robust, randomized available data to adequately evaluate the potential benefits and harms from this drug [hydroxychloroquine],” WHO Director General Tedros Adhanom Ghebreyesus said during an online press conference on Monday.
Information about COVID-19 is rapidly changing, and Scary Mommy is committed to providing the most recent data in our coverage. With news being updated so frequently, some of the information in this story may have changed after publication. For this reason, we are encouraging readers to use online resources from local public health departments, the Centers for Disease Control, and the World Health Organization to remain as informed as possible.
This article was originally published on